The lab currently has several major research directions: olfactory neurobiology and ecology of mosquitoes, Drosophila, black soldier flies and bumblebees; visual processing in mosquitoes; and development of genetic tools in Drosophila and mosquitoes. We use behavioural experiments, live Ca2+imaging, molecular biology, genetics, immunohistochemistry and 3D modelling. And we also do fieldwork!
1. Mosquito olfactory neurobiology
Mosquitoes are important because they transmit deadly diseases such as malaria, yellow fever and Zika. Mosquitoes use their sense of smell to find humans and food, and to avoid harmful substances in their environment. However, we know little about how mosquito olfactory neurons work. Our work focussed of malaria mosquitoes Anopheles gambiae. We study both the aquatic larvae and the terrestrial adults.


We have recently developed transgenic malaria mosquitoes Anopheles gambiae that, for the first time, allowed us to express GFP specifically in their olfactory neurons. Next, we wanted to find out what these neurons do. To start answering this question, we made a mosquito that carries a fluorescent activity indicator GCaMP. When expressed in the olfactory neurons of adult mosquitoes, GCaMP fluorescence showed that these neurons do not respond directly to artificial insect repellents, but instead act by trapping molecules of attractive odorants.
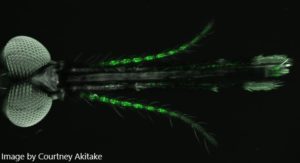
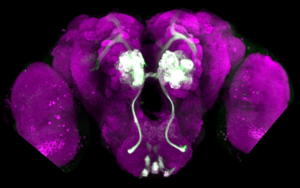
Mosquito larvae also have olfactory neurons and can smell a range of attractive and repellent substances. Thus, it should be possible to control larval behaviour by using olfactory repellents and traps, just like is done with the adult mosquitoes. Olfactory system of larvae is much simpler than that of an adult mosquito, but remarkably little is known about it. We are now using GCaMP to monitor responses to odorants in vivo in the larvae. We are also assaying larval behaviour to see which odorants they find attractive or repellent.
Read more here:
Riabinina O, Quinn M, Whitehead JP (2022) Genetic toolbox approaches in mosquitoes. Cold Spring Harbor Protocols, Invited contribution. doi:10.1101/pdb.top107691 link request free pdf
Wheelwright M, Whittle CR, Riabinina O (2021) Olfactory systems across mosquito species. Cell and Tissue Research, 383, 75–90 link Editorial
Afify A, Betz JF, Riabinina O, Lahondere C, Potter CJ. (2019) Commonly used insect repellents hide human odors from Anopheles mosquitoes. Current Biology, 29, 1-12. link
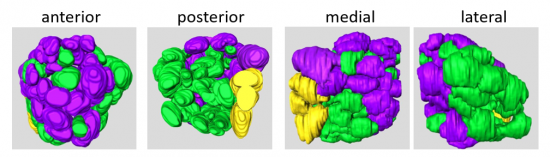
Riabinina O, Task D, Marr E, Lin C-C, Alford R, O’Brochta DA, Potter CJ. (2016) Organisation of olfactory centers in the malaria mosquito Anopheles gambiae. Nature Communications, 7, 13010. link

2. Mosquito vision
This is a new direction in the lab – we were fascinated by the development of larval and adult eyes in the mosquito larvae, and decided to dig in deeper! We have not published on this topic yet.
3. Mosquito ecology
This is another new direction in the lab – or rather in the field! We are focussing on Anopheles mosquitoes that are malaria vectors. We are asking how cuticular hydrocarbons of these mosquitoes evolve, and whether these compounds help the mosquitoes to spread to new areas of the world. Our work has recently been funded by NERC – watch this space for updates soon!
4. Bumblebee olfactory neurobiology and ecology
Another new direction of research, although Lena had worked on bumblebee visual navigation during her PhD. Now we focus on the 10+ species that inhabit Durham and surrounding areas to learn about their perception of flower smells and how it affects their choice of flowers. No papers yet on the topic – watch this space – although we have written a review about bee olfaction.
Read more here:
Gomez Ramirez WC, Thomas NKT, Muktar I, Riabinina O (2023) Neuroecology of olfaction in bees. Current Opinion in Insect Science, 101018 (in press) doi:/10.1016/j.cois.2023.101018 link



5. Olfaction of Black soldier flies
Black Soldier Flies Hermetia illucens, otherwise known as BSF, are heavily used in industry. Their larvae can bioconvert large amount of organic waste into fertiliser, and the larvae thenselves are use as animal food supplements or sources of dietary fat and proteins. However, despite this heavy industrial use, little is known about sensory neuroscience and sensory ecology of the BSF.
Our first BSF project identified chemicals that signal an attractive place to lay eggs to BSF females. These chemicals occur in natural oviposition sunstrates of the flies, and we faschioned them into an artificial chemical mixture of 5 that efficiently attract oviposition. Incidentally, we also identified a mixture of another 5 chemicals that form an efficient oviposition repellent!
There are many future direction we would like to explore from here. We want to test our attractant and repellent in an industrial setting, to work with chemists on an optimal formulation, and to continue investigating how the flies’ brain detect and interprets olfactory cues.
Read more here:
Thomas NKT, Karpati Z, Schmitt T, Riabinina O (2024) A chemically defined oviposition attractant and repellent of Black Soldier Flies (Hermetia illucens). bioRxiv



6. Development of genetic tools
We are interested in developing novel genetic tools that can facilitate neuroscience research in fruit flies Drosophila and malaria mosquitoes Anopheles gambiae. We developed the second generation of Q-system in Drosophila and introduced the Q-system into A. gambiae by genetically labelling olfactory receptor neurons of the mosquitoes.
Our latest project has introduced split-QF into Drosophila and validated its use in a number of proof-of-principle experiments. We also confirmed that split-QF works with split-GAL4 and split-LexA, thus greatly expanding the possible applications of all three systems.
Read more here:
Folsz O, Lin C-C, Task D, Riabinina O, Potter CJ (2022) The Q-system: A versatile repressible binary expression system. Book chapter. In Drosophila: Methods and Protocols (ed: C. Dahmann). Methods in Molecular Biology, 3rd edition, Vol. 2540, 35-78. link order from Amazon request free pdf
Riabinina O, Vernon SW, Dickson BJ, Baines RA. (2019) Split-QF system for fine-tuned transgene expression in Drosophila. Genetics, 212, 1, 53-63. link
Riabinina O, Task D, Marr E, Lin C-C, Alford R, O’Brochta DA, Potter CJ. (2016) Organisation of olfactory centers in the malaria mosquito Anopheles gambiae. Nature Communications, 7, 13010. link
Riabinina O, Potter CJ. The Q-system: A versatile Expression System for Drosophila. In Drosophila: Methods and Protocols (ed: C. Dahmann) (Methods in Molecular Biology, Vol. 1478, 53-78) link
Riabinina O, Luginbuhl D, Marr E, Liu S, Wu MN, Luo L, Potter CJ. (2015) Improved and expanded Q-system reagents for genetic manipulations. Nature Methods, 12, 219-222 link





Past funders:






